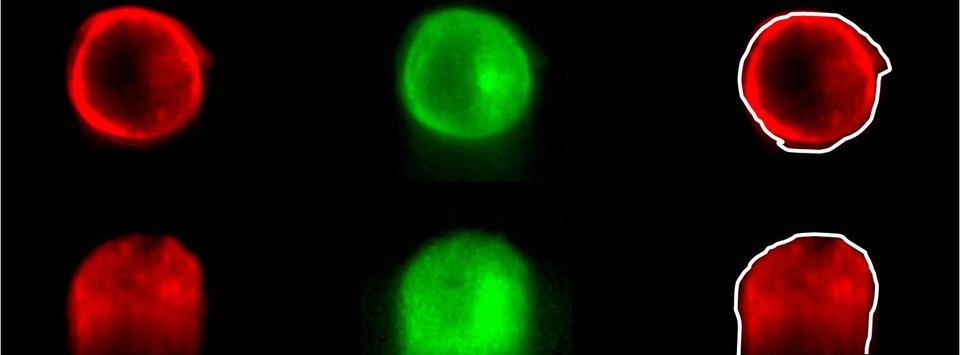
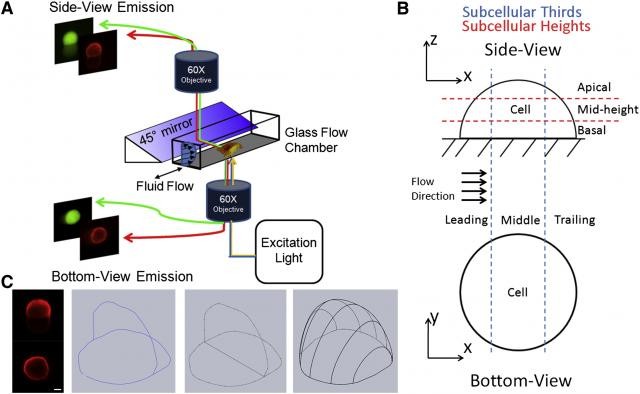
Single Cell Studies and Cytoskeleton Mechanics in Quasi-3D
Osteocytes are three-dimensional, ellipsoidal, mature bone cells encased in mineralized extracellular matrix. It has been hypothesized that the dominant loading mechanism of the mechanosensing osteocytes is fluid shear stress resulting from the deformation of bone. Recent experimental and theoretical evidence suggest that the ellipsoidal cell body may be less sensitive to fluid shear than the slender cellular processes. However, for adherent cells under fluid flow, the interactions between the flow field, the cell, and the substrate are dynamic and complex. The conventional top-to-bottom view microscopy of cell deformation is insufficient to characterize the intracellular deformation of the adhered cells at their apical surface (cell body) and at their basal surface (cellular processes and dendrites) under flow.